#97 Lithium, Lithium, Everywhere, and None to Use for Fusion Reactors
Return to the Fusion Fuel Main Page
By Steven B. Krivit
Jan. 8, 2022 (Updated Jan. 27, 2022)
Earlier this year, New Energy Times reported that one of two essential fuel sources for nuclear fusion — the hydrogen isotope called tritium — does not exist on Earth as a natural resource.
New Energy Times has recently learned, however, that the alternate fueling strategy, to make tritium from the lithium-6 isotope, is equally flawed. Lithium-6 is not commercially produced in industrial quantities anywhere in the world except by military organizations. It is a controlled material, used in thermonuclear bombs.
For decades, fusion proponents have told the public and decision-makers that the fuel for fusion is abundant, inexpensive, and universally available, despite the fact that tritium and lithium-6 are none of these. Here are two examples of such claims:
Thomas Klinger
In 2017, Thomas Klinger, the scientific director of the Wendelstein 7-X Project at the Max Planck Institute for Plasma Physics, in Munich, Germany, gave a TEDx lecture in Brussels. Here’s what he said about the fuel for fusion reactors:
“It is abundant, enough fusion fuel for millions of years – and is accessible to everybody. So nobody owns the fusion fuel. The machines are expensive, but the fuel cost is essentially zero.”
Michel Laberge
Michel Laberge is the founder of General Fusion Inc. Here’s what he said in a 2014 TEDx lecture in Kansas City:
“The fuel that you need for fusion, you can extract it from the ocean. You can extract the fuel from the ocean for one-thousandth of a cent per kilowatt-hour. If the whole planet was run on fusion, there would be enough fuel in the ocean for 2 billion years. So there’s enough fuel, and it’s nice, and it’s clean, and it’s fantastic.”
Tritium
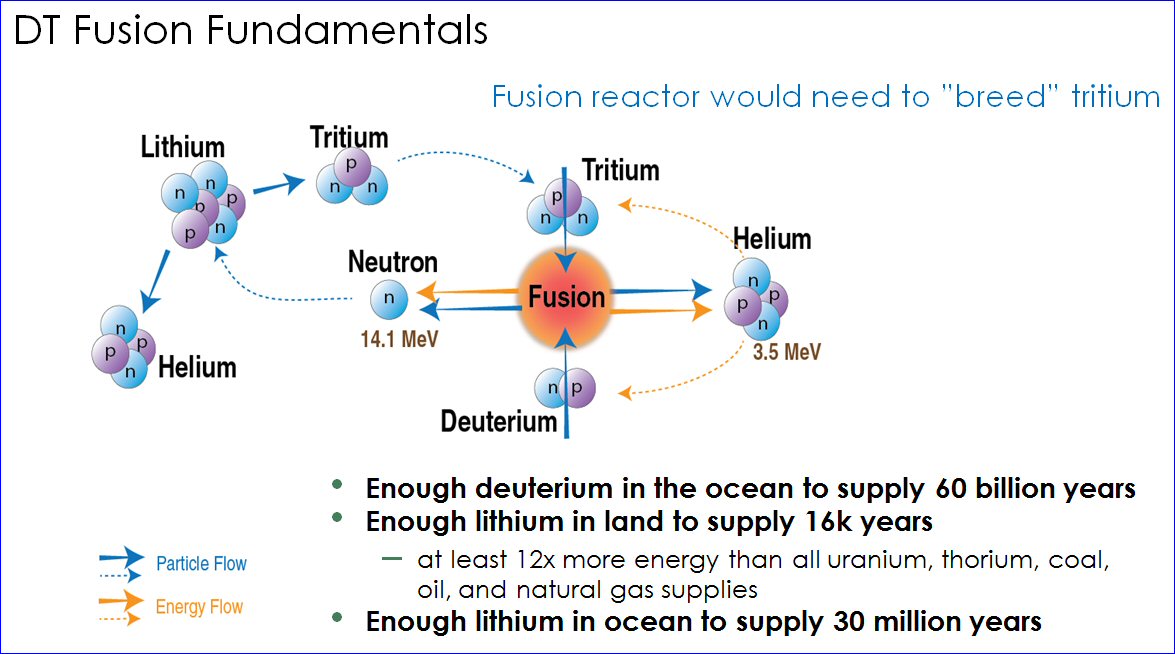
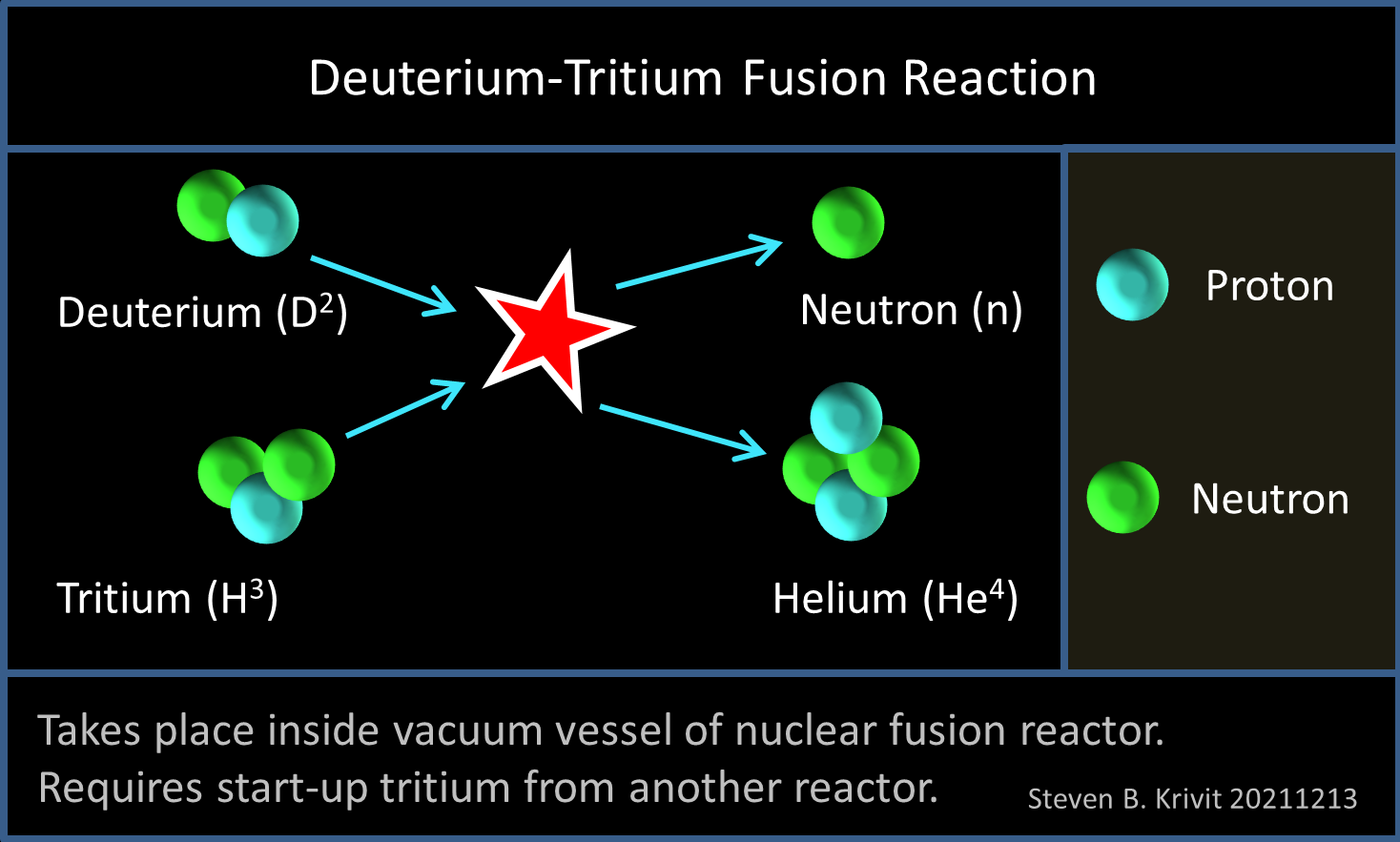
Most of the world’s planned fusion reactors are designed to use a 50-50 mixture of two forms (isotopes) of hydrogen: deuterium and tritium. Like normal hydrogen, they each have one proton in their nucleus. But deuterium also has a neutron, and tritium has two neutrons.
Although other fusion fuel combinations are possible, scientists know that this 50-50 mixture of deuterium and tritium (DT) is the most effective fusion fuel combination. They’ve known this for at least 50 years.
Deuterium-tritium fusion reactors require roughly equal quantities of deuterium and tritium. Tritium is priced starting at $30,000 per gram. It is available primarily from only one country, Canada. It will be produced for only the next 40 years. The ITER reactor in France will consume most, if not all, of the worldwide tritium inventory.
Tritium is a government-controlled, radioactive material produced by an aging fleet of specialized nuclear fission reactors that will not be replaced. All of them are scheduled to be decommissioned by 2060, with no known replacements planned. Because tritium begins to undergo rapid radioactive decay as soon as it is produced, any stockpile will be rapidly depleted by decay into helium-3.
On the other hand, deuterium is equally accessible to everyone, everywhere, and is not difficult to extract from water.
Experienced fusion scientists have known for at least 50 years that tritium is not and will not be available as a primary fuel source for fusion reactors. For more details on why tritium cannot be a long-term fuel source for nuclear fusion, please see this article.
Lithium
Instead, fusion scientists have planned to make tritium from a natural resource that is relatively abundant: a soft, lightweight metal called lithium. But this path has significant technical, logistical, and legal problems.
They have known that, in the laboratory, the lithium-6 isotope can be bombarded with neutrons to make tritium. But nobody knows whether this will work well enough in a reactor. It must be tested before any conclusion can be drawn about whether fusion can be developed into a practical source of energy. If the test fails, the quest for fusion is over. Scientists expect to run several small-scale tests in ITER in perhaps 20 years. Scientists expect to run the first full-scale tritium breeding test in the EU DEMO reactor in 30 or 40 years.
Lithium-6 is available in scientific quantities of grams to everyone. It is also a special material used for thermonuclear weapons. Fusion reactors will need Lithium-6 in quantities of tons, which is produced by and available only to military organizations.
Using Lithium to Breed Tritium
It is scientifically possible to make, or breed, tritium inside a fusion reactor, using an induced fission reaction. However, some of the fusion scientists have been conflating the fusion and fission processes.
In January 2019, scientists at the Massachusetts Institute of Technology (MIT), in collaboration with Commonwealth Fusion Systems (CFS), published a brochure about a fusion reactor they intend to build called SPARC. The reaction diagram they provided in the brochure showed deuterium and lithium-6 as the raw materials. According to their diagram, the reaction between deuterium and tritium would produce helium and a neutron. Then, the neutron from the first reaction would react with lithium-6 and produce tritium.
Such a depiction might be confusing and appear to use circular logic. In fact, it does. As depicted, this is an imaginary reaction process and inaccurately represents what would happen inside a fusion reactor.
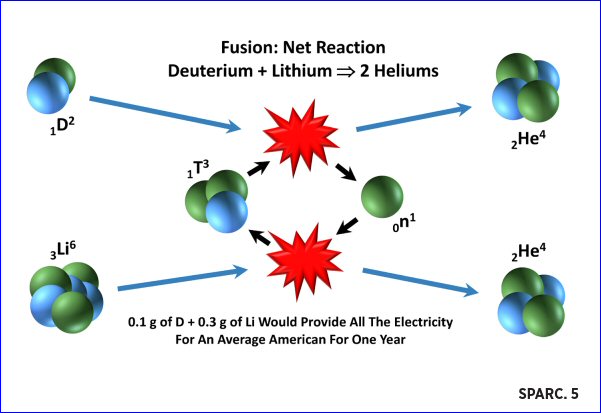
MIT/CFS SPARC reactor reaction diagram
The MIT/CFS scientists are not the only ones who are conflating the primary and secondary reactions. In the summer of 2021, the Princeton Plasma Physics Laboratory offered an introductory course on nuclear fusion. Cami S. Collins, a fusion scientist at the Oak Ridge National Laboratory, provided the introductory course. In her slides for the course, she, too, displayed a similar, circular reaction equation.
She explained, in a video recording of her class, “You take the neutron, and it reacts with lithium, and that produces tritium. So a fusion reactor would need to master this process of breeding tritium; it’s not proven or trivial.”
Her disclaimer was well-placed. Her optimism about the abundance of lithium, however, was not, and I will get to that in just a moment.
Primary and Secondary Reactions
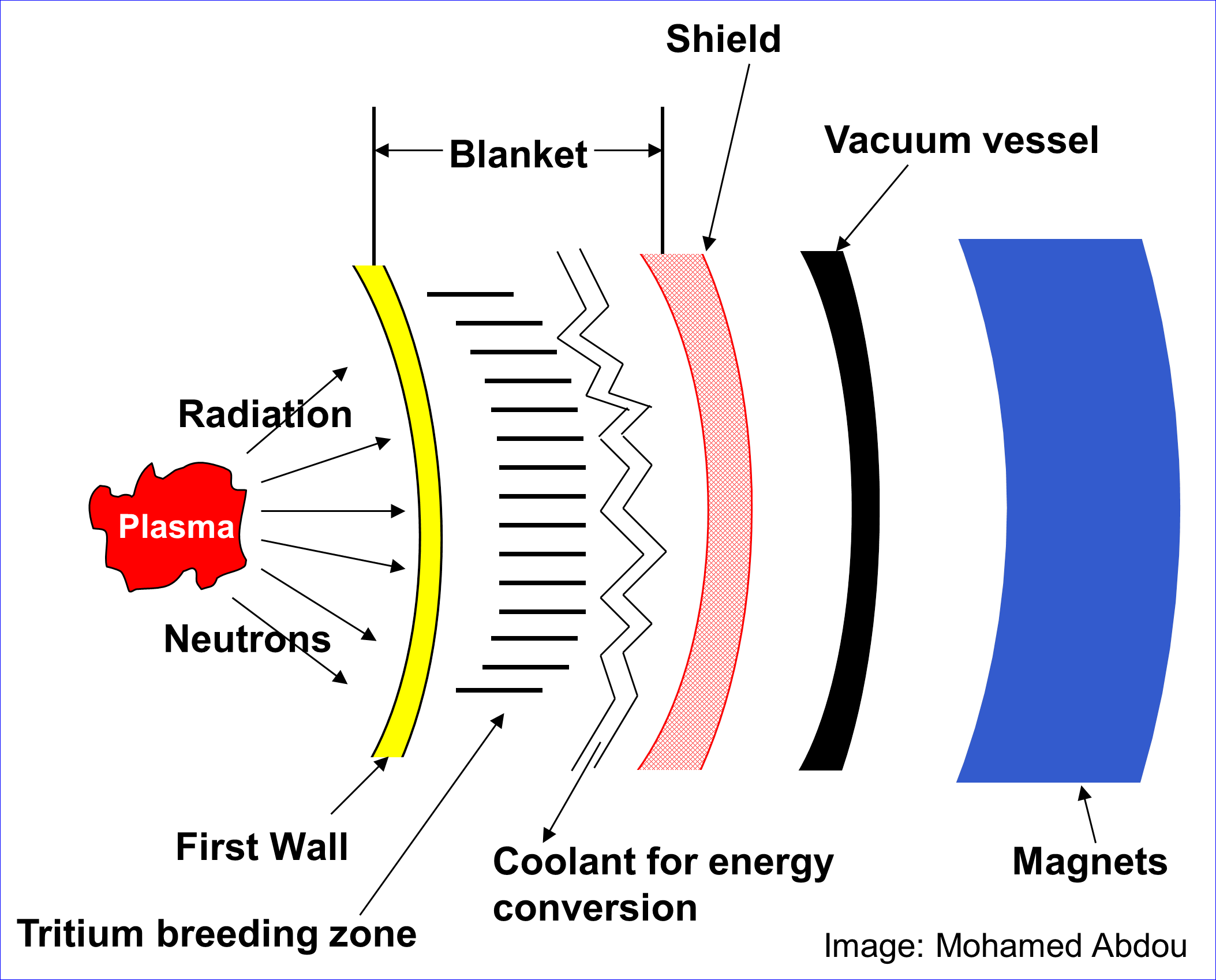
The reaction diagrams by Commonwealth and by Collins misleadingly conflate the primary and secondary reactions. The primary reaction is the DT fusion reaction. The fusion reaction would take place within the plasma inside a fusion reactor. The fission reaction would take place beyond the plasma, inside the first wall in the tritium breeding zone. The two reactions would be physically isolated from each other.
For starters, even though a DT fusion reactor might produce its own tritium, it would need to have tritium from another source when it starts. It would have to receive a shipment of tritium produced by another fusion reactor or by a specially configured fission reactor. Some fusion proponents claim that a fusion reactor could possibly start up without an external tritium supply. This is true. But Kovari et al. have refuted this consideration because, as they reported, the operating and capital expenses for a fusion reactor that produces no electricity and only tritium is in the billions.
One of the products of the DT fusion reaction is a neutron. The neutrons would be thermalized, that is, converted into heat. This heat would, theoretically, be used to generate electricity in a commercial fusion reactor. Thiéry Pierre, a plasma physicist and senior scientist at the Centre National de la Recherche Scientifique, in Marseille, France, explained that some of the neutrons would also be used to breed tritium. But there’s a complication. These neutrons must first be used to produce more neutrons for the whole process to work. This is why it is absolutely necessary to use a neutron multiplier material to produce enough tritium. The optimal material to use for this is beryllium.
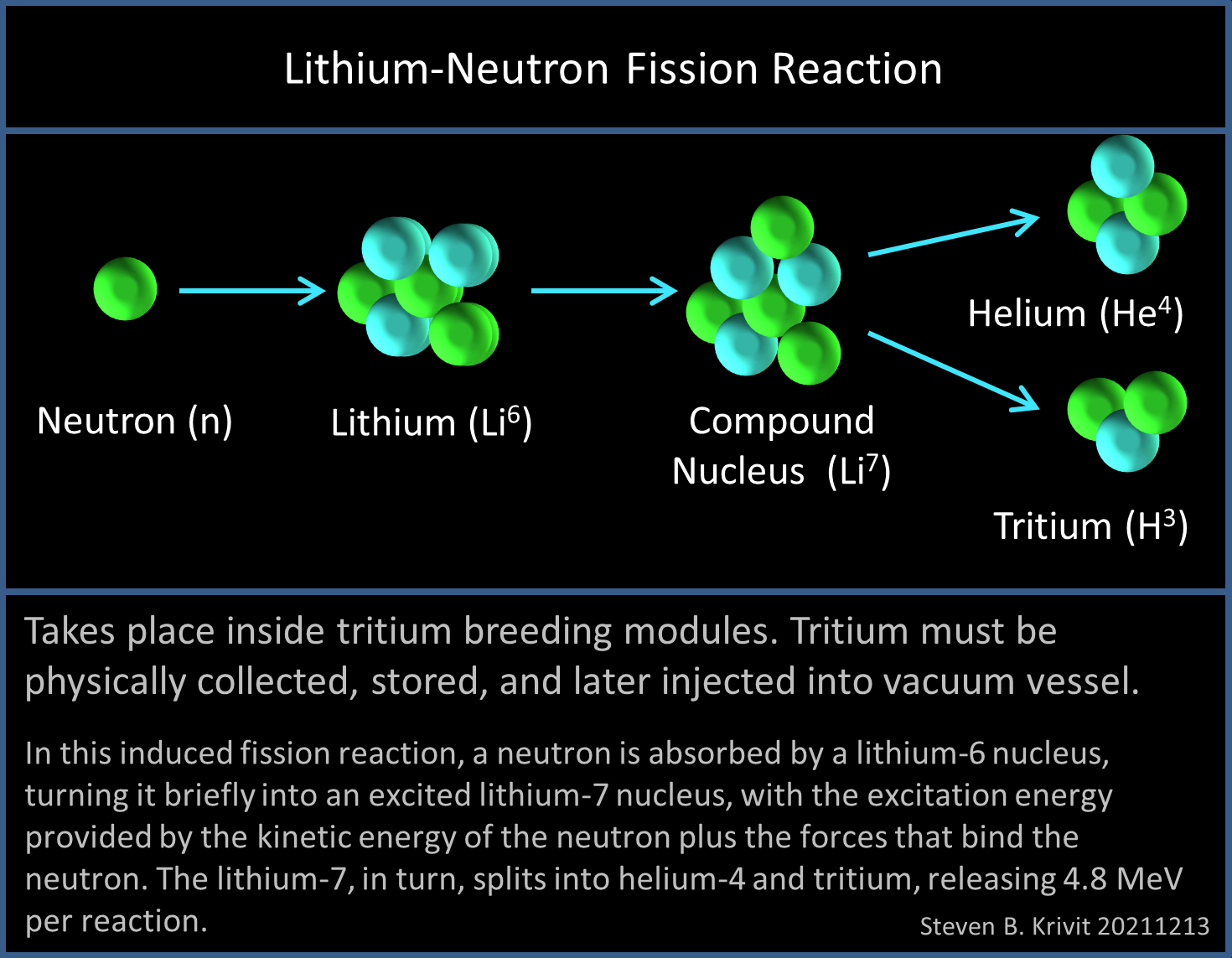
A neutron would react with the lithium-6 isotope contained in the tritium breeding zone, temporarily forming a lithium-7 isotope, then fissioning into helium and tritium. Separately, an extraction process would be required to collect the produced tritium. It would be stored and later injected into the vacuum vessel. Thus far, no reactors have been built with a tritium breeding blanket, and the idea has not been experimentally tested.
ITER is planned to be the first reactor to perform such tests but only on a very small scale. The tritium-breeding modules in ITER will not be able to produce more than 1 percent of ITER’s tritium consumption. Full-scale tests of tritium breeding in a fusion reactor are not planned until the DEMO-class reactors are built, perhaps in 40 years.
It is not sufficient for the process to make tritium at the same rate as a reactor would consume tritium. As a result of limitations of tritium recovery as well as operational downtime, the breeding rate of produced tritium must be higher than the breeding rate of consumed tritium. This is called the tritium breeding ratio. If the ratio is not high enough, the fusion reactor would not be tritium self-sufficient. If there are no more fission reactors to provide external sources of tritium, a fusion reactor would soon run out of fuel. Objective fusion scientists have paid serious attention for many years to the question of whether fusion reactors will be able to produce a sufficiently high tritium breeding ratio.
If this ratio can’t be achieved, then the quest for fusion on Earth — at least through DT fusion — will terminate without success.
In a peer-reviewed paper published last year, prominent experts in tritium breeding concluded that, based on known physics and state-of-the-art technology, fusion reactors following ITER will not be able to breed enough tritium to be self-sufficient.
In contrast to their peers who sugar-coat or ignore the serious known challenges with fusion research, the authors of this paper — Mohamed Abdou, Marco Riva, Alice Ying, Christian Day, Alberto Loarte, L.R. Baylor, Paul Humrickhouse, Thomas F. Fuerst, and Seungyon Cho — were transparent and blunt. For full details and references on their paper and the broader tritium issue, please see this news article.
Two Isotopes
Natural lithium, mined from the Earth, contains two stable isotopes: Lithium-7 and Lithium-6. Lithium-7 has 3 protons and 4 neutrons; Lithium-6 has three of each. Natural lithium contains 92 percent Lithium-7 and only 7.5 percent Lithium-6.
During her introductory course on nuclear fusion, Collins told her students that sufficient lithium can be mined from land to provide fusion fuel for 16,000 years. She said there was enough lithium fuel in the ocean to last for 30 million years. But she didn’t distinguish between the two isotopes. When lithium is used as a chemical fuel — for example, in batteries — the isotopes are irrelevant; natural lithium will suffice.
But when lithium is used in nuclear reactions, the two isotopes behave differently. Technically, both Lithium-7 and Lithium-6 can produce tritium when bombarded with a neutron. But the isotope needed for use in DT nuclear fusion is Lithium-6.
The primary reason lithium-6 is needed is because the probability of the Li-6+neutron reaction, known by physicists as the “cross-section,” is much higher than the Li-7+neutron reaction. As such, the tritium yield from the lithium-7 reaction will not breed enough tritium to make a fusion reactor tritium self-sufficient. That means it will consume and lose more tritium than it produces.
A secondary reason is that, in the course of making tritium, the Lithium-6 reaction is exothermic, meaning it releases 4.8 MeV of energy per reaction and contributes to recoverable heat in the blanket and, therefore, to reactor energy production. The Lithium-7 reaction, on the other hand, is endothermic: It consumes 2.5 MeV energy per reaction. The primary energy production in DT fusion reactors will come from the 14 MeV neutron, emitted in each fusion reaction.
The only problem is that Lithium-6 is not commercially available in the quantities needed for fusion reactors.
No Lithium-6 in North America
The Lithium-6 and Lithium-7 isotopes require separation, what is called enrichment, which increases the percentage of the desired isotope. A scientific paper from the MIT/CFS fusion team explains that they will need Lithium-6 enriched from its natural state of 7.5 percent to 90 percent.
Starting in 1955, one plant in the U.S. operated at the Oak Ridge National Laboratory in its Y-12 National Security Complex in Tennessee. It used what was called a “COLEX” process, which has since been banned in the U.S., according to a 2013 report from the U.S. Government Accounting Office:
Y-12 experienced several problems with the COLEX process, including equipment failures, worker exposure to mercury, and mercury contamination of the environment. Y-12 shut the COLEX facility down in 1963 and has not operated it since then.
The May 2012 report #UCBTH-12-005 from the Department of Nuclear Engineering at the University of California, Berkeley, said that “large quantities of mercury were lost at Oak Ridge to wastes, spills, and through evaporation.” The report said, “Cleanup remains extremely difficult and expensive, both in initial costs and environmentally.”
According to the November 2020 report #DOE/EA-2145 from the National Nuclear Security Administration, a new lithium processing facility is planned for construction at Oak Ridge, with operation beginning in 2030. The report describes only the environmental assessment of the new facility; it does not describe the method that will be used to separate the lithium isotopes.
So why did Oak Ridge have a lithium processing plant? And why is it building a new plant? Pierre explained to New Energy Times why the military needs enriched Lithium-6:
The isotopic separation of Lithium-7 and Lithium-6 is controlled because Lithium-6 is the one that makes it possible to produce thermonuclear bombs.
The U.S. military has a stockpile of Lithium-6, left over since 1963. The stockpile should be able to replenish existing nuclear warheads through 2030, according to the reports.
What About Russia or China?
One way to look for possible sources of Lithium-6 is to look for sources of Lithium-7. Where you find one isotope, you will find the other.
According to the 2013 GAO report, Lithium-7 is required for the majority of the U.S. nuclear fission reactors, called pressurized water reactors. The 2013 report explains that the Lithium-7 isotope is needed to reduce the rate of corrosion of pipes and other infrastructure in such reactors and to prevent them from failing.
The authors of the report wrote that “13 percent of our nation’s electricity is produced by 65 nuclear power reactors that rely on enriched lithium hydroxide—a chemical that is produced and exported only by China and Russia.” The GAO report expresses great concern about risks to the supply of Lithium-7:
China and Russia produce lithium-7 as a byproduct of enriching lithium-6 for their nuclear weapons programs, according to a DOE official, much like the United States previously did. There is no domestic production of lithium-7, and little is known about the lithium-7 production capabilities of China and Russia and whether they will be able to provide future supplies.
According to the U.C. Berkeley report, the Chinese enrichment facilities rely on the same mercury-based enrichment method that was banned in the U.S.
According to a March 2017 report by the Institute for Science and International Security, in Washington, D.C., North Korea has a lithium processing plant as part of its nuclear weapons program.
No military producer of Lithium-6 is going to offer Lithium-6 for sale to industry, even if international law allowed the export.
No Industrial Quantities of Commercially Available Lithium-6 in the World
One research group, at the Karlsruhe Institute of Technology in Germany, has closely examined the lack of an available source of Lithium-6. The lead scientist in the group is Thomas Giegerich.
In Dec. 2019, Giegerich, along with Katharina Battes, Jonas Caspar Schwenzer, and Christian Day, published a peer-reviewed paper and explained that a fusion power plant would need approximately 50 tons of pure Lithium-6 per gigawatt of electricity produced. To their knowledge, there is no facility in the world that can do this:
The need for enriched Lithium-6 in large quantities asks for an isotope separation process that allows a minimum output of several tons of fusion grade lithium (that is, lithium with an isotopic composition that can directly be used in the blankets) per full power year. As far as we know, no facility is available world-wide that could satisfy this demand, and it is also not straightforward to build such a plant. Hence, a suitable process has to be developed, which poses a special challenge for process engineers: Isotope separation is usually very energy demanding and complex, leading directly to large investments.
The Future of Fusion
In the December 2019, paper, Giegerich and his colleagues reviewed a variety of lithium isotope separation processes. They did not find an existing process that, overall, was better than the COLEX process. Instead, they proposed a new mercury process that they believe will be more environmentally friendly than the old COLEX process.
“In this paper, we will describe only the development of an analytical method and a first lithium enrichment experiment,” the authors wrote.
The single experiment they performed indicated that their method could work. However, their focus was the development of their analytical methods and a confirmation of their measurement techniques.
If their process is scientifically and independently confirmed, the authors explain, it would take about 20 years to develop a suitable industrial-scale lithium enrichment facility.
It’s been two years since that paper published. I’ve been unable to locate a subsequent paper from the researchers reporting even their first group of enrichment experiments. I sent e-mails to Giegerich and Day asking for an update, but they did not respond. I called Giegerich and left a voicemail, but he did not respond.
The fate of the worldwide plans for DT fusion is contingent on a method of separating Lithium-6 that 1) is environmentally benign, 2) can be performed at industrial levels, and 3) will be permitted despite proliferation restrictions. No such method exists.
Dead End for DT Fusion?
The largest, most scientifically credible DT fusion project that would breed tritium is the planned EU DEMO reactor. The project is in the design phase and is managed by the European Commission’s EUROfusion organization.
In a 2016 slide presentation, Giegerich and co-authors wrote that the “unavailability of lithium enrichment facilities that could meet [the EU] DEMO requirements is a threat to the success of fusion.”
Tony Donné is the programme manager for EUROfusion. On Dec. 17, 2021, I sent him an e-mail and asked where he expects to obtain Lithium-6 for the EU DEMO. He wrote back the same day with no explanation, just a link to the Giegerich et al. paper I describe above.
Publicly, Donné and his organization say that making tritium onsite, inside a fusion reactor, “would be advantageous because tritium is radioactive, with a short half-life, and so is difficult and expensive to obtain.”
The EUROfusion Web page does not explain that industrial quantities of Lithium-6 are unavailable.
Robert Arnoux, writing for the ITER organization, said that obtaining “tons of the precious isotope will require processing by way of well-established isotope separation techniques.” The only well-established industrial-scale technique is the COLEX process, now banned in the U.S and possibly elsewhere in the world.
A now-deleted European Commission Web page said that a “1 GW fusion plant will need about 100 Kg of deuterium and 3 tons of natural lithium to operate for a whole year, generating about 7 billion kWh, with no greenhouse gas or other polluting emissions.” Three tons of natural lithium contains 200 kilograms of Lithium-6.
Robert Arnoux, in the public relations office of the ITER organization, wrote that a 1 GW electric reactor will consume 500 kilograms per year.
Alexander M. Bradshaw, a physical chemist at the Max Planck Institute for Plasma Physics, wrote that a 2 GW thermal output reactor, which is equivalent to a 0.8 GW electric reactor, will require 300 kilograms per year. Therefore, the numbers are all within the same order of magnitude. Bradshaw also explained that the initial loading of Lithium-6 into reactors will require, depending on the two proposed methods, 22,000 kilograms of 90% enriched Lithium-6 or 9,000 kilograms of 36% enriched Lithium-6.
Lithium From Seawater?
Can lithium for fusion fuel be obtained from seawater? Today, lithium is mined from the earth and extracted from brine pools. The world is already seeing supply-chain resource conflicts for the lithium needed for batteries.
Fusion scientists say that lithium can also be obtained from seawater. For perspective, some fusion scientists also say that helium-3, as an alternate fusion fuel source, can be mined from the moon. Both statements are true. Neither statement accounts for the cost to obtain the particular fuel.
No economical method exists to harvest natural lithium from the ocean. That is because the concentration of natural lithium in seawater is dilute: roughly 0.2 ppm. Until and unless an economical method of ocean harvesting is developed, fusion fuel will need to come from conventional sources, which will only add to the current geopolitical lithium resource conflicts.
Using lithium for fusion will be even less practical than using it for batteries, because only about 7.5 percent of the lithium in that 0.2 ppm contains the needed lithium-6 isotope. Additionally, ocean-harvested lithium-6 is subject to all of the same problems as discussed above (lack of processing plants, proliferation risks, etc.) that mined lithium is.
Castles in the Air
We already know that fusion scientists have misrepresented planned power gain for reactors far out of reality.
Now we know that fusion scientists falsely represented that fuel sources for DT fusion are abundant, inexpensive, and universally available.
Even if a new environmentally benign commercial facility capable of producing tons of Lithium-6 per year is built and completed 20 years from now, the cost to initially load a reactor with Lithium-6 likely will be in the tens of millions of dollars. The yearly fuel replacement cost likely will be in the millions of dollars. Even if an industrial source of Lithium-6 becomes available, DT fusion reactors will always need tritium to start up; there is no cost-effective alternative for the startup tritium. This issue is compounded by the fact that, if ITER goes as planned, it will use up almost the entire worldwide inventory of tritium.
We now know that tritium does not exist as a natural resource. We know that, within a few decades, tritium will no longer be produced and that it can’t be inventoried long-term. Therefore, we know that, soon after mid-century, commercial tritium will not be available.
Some fusion scientists have claimed that DT fusion reactors will be able to produce sufficient amounts of tritium by breeding it from lithium. Other fusion scientists say there is no known physics or technology that will allow this. We now know that there is no industrial source of lithium-6.
Thus, the claim that fusion will produce “commercially relevant energy” from “a resource as common as a glass of seawater” is the modern-day equivalent of snake oil.
Author’s Note: I wish to thank plasma physicists Daniel Jassby and Thiéry Pierre for their assistance with this article.
Note: This article was updated on Jan. 27, 2022, to add the Lithium From Seawater section