Demystifying Nuclear Radiation
The nuclear power plants at the Fukushima, Japan, site are experiencing serious failures. These failures pose hazards to life and the environment, as well as radiation risks to the rest of the world.
Mainstream science media are beginning to focus more on the facts and feed less on people’s fears, but essential information is lacking.
Scientific American has a good article that provides a general perspective on the risks but doesn’t help readers understand radiation.
CNN has an informative news article on nuclear radiation hazards but fails to explain this crucial concept.
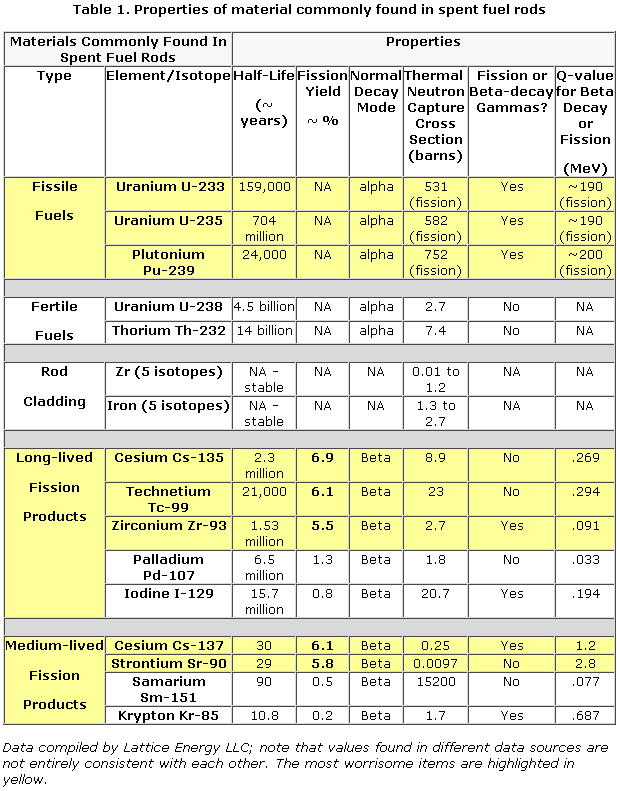
There are several common types of radiation emissions. Georgia State University has a good online reference.
Alpha particles, which are the nuclei of helium atoms, can travel only an inch or two in air and can be stopped by a piece of paper, but if they enter the body, they can be deadly.
Beta particles, which are essentially electrons, which don’t travel much farther, can be stopped by a thin sheet of metal.
Gamma rays, which are very short-wavelength electromagnetic radiation, can go through several inches of steel or lead. According to the EPA, they can travel thousands of miles in air.
Most nuclear reactions also emit neutrons. Free neutrons will be half-dead in 13 minutes, so these don’t pose any remote hazard. Neutrons can, however, turn non-radioactive elements into radioactive ones.
How can nuclear radiation from Japan reach Southern California, for example, if radiation can travel only a few thousand miles?
Radiation itself doesn’t travel that far, but radioactive elements do.
Microscopic or, in some cases, macroscopic physical particulate from inside the reactor fuel escapes into the atmosphere during a nuclear incident when fuel enclosures melt.
The particulates get released through vented gasses or expelled during explosions; the particles that travel the farthest are submicron-sized. Some radioactive elements in the vapor phase are made in reactors and released, but these are diluted in the atmosphere and dispersed quickly, and they pose little remote risk.
These gases, vapors and particulates can be made of a variety of materials that could be radiation-emitting elements, such as iodine, krypton, cesium, uranium or plutonium, or they could be elements that are not normally radioactive but may have become so as a result of the fission process and neutron interactions inside the reactor.
These elements emit one or more of the radioactive emissions: alpha, beta, neutrons and/or gamma rays.
So when Fox News says that “there are two types of radioactive material, and the crew members [of a Navy helicopter crew] in Japan were exposed to radioactive particles (alpha and beta) which resemble dust,” this description is misleading.
The visible particulate, the radioactive material, may resemble dust, but the alphas, betas and gammas themselves are nuclear particles that come out of the radioactive material and are invisible to the naked eye.
Knowing the source of such radiation would be meaningful. Each radioactive element emits radiation in different intensities for different durations. Depending on which element and sub-species of elements are emitted, people can be better informed and prepared to respond, if necessary.
The short- or long-term consequences vary. Some radioactive contaminates wash off with water and are half-dead in 30 years. Others last 10 million years or longer.
If scientists detect abnormally high levels of radiation in the atmosphere, they should be able to explain which elements are emitting them, because each radioactive element emits unique signatures (that is, a specific combination, intensity and duration of alpha, beta and gamma ray emissions).
If remote radiation threats pose real local risks, Fox News and CNN will broadcast the information.
Fossil fuels aside, if 45.7 percent of worldwide electrical power comes from nuclear plants, and one-tenth of 1 percent comes from solar energy, experts have to find a way to make nuclear fission work or find another type of nuclear solution.